Cryopreservation
Everyone wants live shipping for their cells but the traditional method is cryopreservation. For many research laboratories this method has proven to be a cheap, reliable and effective way to maintain cell stocks. Frozen aliquots are either brought up in large volume of media to rapidly dilute the toxic effects of DMSO or cells are quickly washed via centrifugation prior to seeding. This storage can be accomplished in a laboratory by simply maintaining a dewar of liquid nitrogen and scheduling regular liquid nitrogen deliveries.
Cellular therapies intended for human administration have become a reality. As a result, the costs, limitations, and drawbacks of traditional laboratory cryopreservation, as adapted for human cell therapies, have become clear. The finished cell product, having just passed the final QC check, now has to undergo a freeze-thaw cycle before being shipped to the patient treatment site. For many non-hematopoietic cells this can lead to an acceptable <10% loss of viability, but for many fragile blood cell therapies, a freeze-thaw cycle can lead to losses of 15-25% of cells. In addition, it is unclear which cell therapies will require a final wash process to remove cryoprotectant prior to administration.
Limitations of Cryopreservation
The need for cryo-processing limits autologous cell harvesting and final administration to academic centers of excellence that have the facilities and expert staff to cryopreserve samples and/or wash the final product under GMP conditions. This limits the geographical roll-out of a commercial product and adds tremendously to the cost of goods. Moreover, reliably shipping a cryopreserved product worldwide often requires >3 days transit time, which dry ice alone cannot reliably support, thus most cell therapies will require complex, sophisticated and extremely expensive cold-chain logistics systems that utilize liquid nitrogen shippers. All of this adds obscene cost, complexity, and risk to an already complex process and further limits the geographical market.
These problems have been written about extensively and is now well understood. The problems and costs of cryopreservation are so well recognized that in their Technology Roadmap to 2025, the National Cell Manufacturing Consortium (NCMC) identified the following topics as high priorities needed to support widespread adoption of engineered cell therapies:1

- Improve understanding of cell responses to cryopreservation and thawing interactions to inform process design.
- Identify better containers (e.g., smart or reusable packaging) for cell preservation to decrease shipping costs
- Identify shipping and storage alternatives to cryopreservation

Many industry experts have gone on record stating that for autologous cell therapies to be economically viable and succeed in the long-term, a reliable non-cryo method of cell preservation MUST BE developed and integrated into the production.
Live, Fresh Cell Shipping
Ideally, all cell therapies would be shipped live, fresh and viable, to any location worldwide, in the solution already validated for administration. Until now this has been impossible because the cell therapy industry only had flasks, bags, tubes and cryovials to choose from. For most cells, shipping live in one of these containers is not practical. With gas-permeable bags, there is no gas control, thus cells face the dual threat of hyperoxic gas exposure and a critical loss of CO2, and thus loss of pH control. In addition, gas-permeable containers like bags suffer from loss of volume due to evaporation, which changes dissolved concentrations and osmolarity. Dr. Men Wei recently gave a talk at the Cell Series Conference in London highlighting the failure rate of traditional bags following freeze-thaw. He reported that in some scenarios bag failure rate can be as high as 1%, an exceedingly high and unacceptable rate of loss when considering the cost of goods and scarcity for a finished autologous cell therapy product; so gas-permeable containers are no good for live cell storage and transport.
Sealed containers like conical tubes, cryo vials and sealed flasks are extremely limited in supporting live cell transport. Although they are functionally-closed, thus preventing dehydration and maintaining osmolarity, since there is no mechanism for gas supply the cells will rapidly die from hypoxia-induced apoptosis. So bags don’t work and sealed tubed and flasks are no good either. These traditional live shipping containers can rarely provide more than 3 days of reliable live cell transport. For some domestic shipping this may be sufficient when everything goes well, but in the event of delays due to airline problems, worker strikes, weather or customs delays, this limited window of time would surely spell disaster for your valuable and unique cell therapy product; this is unacceptable.
The Ideal Container for Live Cell Storage and Shipping
An ideal cell preservation technology should work over a broad range of temperatures, (4-37°C), maintain low dissolved oxygen levels, and retain CO2 and control pH in order to preserve the cells in a gentle and natural state of metabolic dormancy. This state of dormancy must be easily reversible and must not require any special wash or rinse steps prior further processing. Technology that provides these specific features and functionality would be considered the “holy grail” of cell product transport. These features are exactly what the Petaka G3™ cell culture cassette provides.
The Petaka G3™ features an integrated, microchannel-based gas diffusion control system that maintains cells at physiologic oxygen levels (2-4% pO2) even when exposed to open air (21% pO2). As cells release CO2, the microchannel restricts outward diffusion thus maintaining the equivalent of 5-7% supplemental CO2 internally. These features combine to make the Petaka G3™ function like a closed, portable mini-incubator system…no hypoxia chambers or supplemental CO2 required!

A review by Robinson, et al 2, details the challenges and risk of traditional cryoprotection and the advantages of low temperature or ambient, live cell shipping by gentle cell pausing. In the Petaka G3™, cell pausing is achieved in a way that reliably provides 7 to 10-day non-cryo cell preservation for almost every cell type. The process is simple. Once the cells in the Petaka G3™ equilibrate to low oxygen while in culture simply remove the Petaka cassette from the 37-degree incubator to store or ship cells….that’s all there is to it! In this special state, characterized by low oxygen, reduced temperature, and slight reduction in pH (6.9-7.0), the cells “go to sleep”. At this point population doubling time increases many fold and metabolic activity (and waste production) drops many-fold. Experiments show that cells are happy in this condition for many days or weeks. Once the cells are warmed again, metabolism instantly resumes with no damaging effects on the cells.
Because the Petaka G3™ is virtually closed, loss of liquid due to evaporation is negligible even up to 30-days. O2, CO2 and pH levels are also perfectly maintained. This unique combination of oxygen, CO2, and pH control, with no loss of medium from evaporation now makes the possibility of worldwide, live cell shipping a reality.
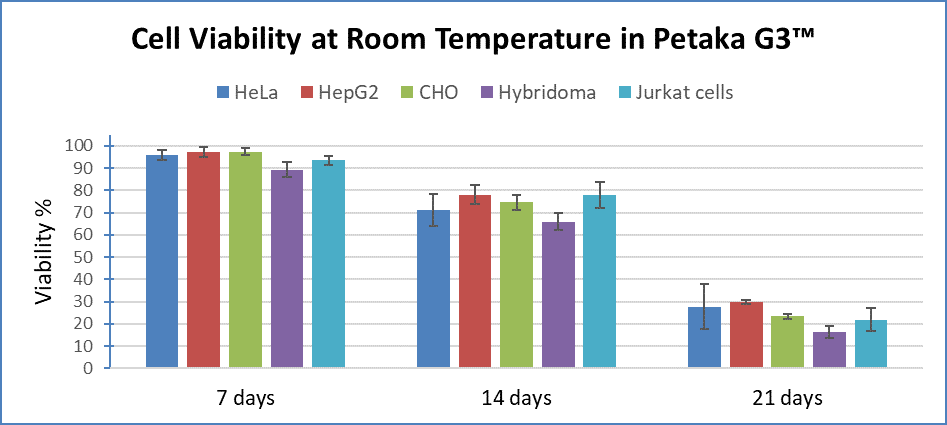
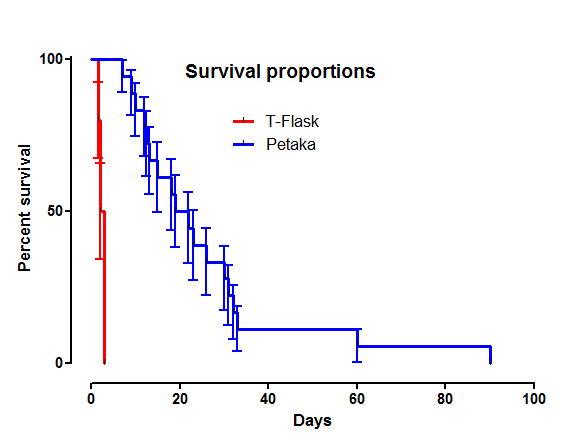
This short review is meant to highlight the urgent need for non-cryo cell preservation methods. Industry groups admit it is necessary for widespread and affordable cell therapy adoption. However, until the development of the Petaka G3™, no container technology existed that could reliably maintain non-cryo cell preservation beyond 4 days. All that has now changed!
If you have been tasked with developing a long-term, non-cryo solution for your cell transport, I recommend exploring the many benefits of live cell shipping using the most advanced cell preservation technology in the world. Many customers worldwide have already incorporated the Petaka G3™ into their GMP laboratory operations. You should give it a try too.

REFERENCES
- Achieving Large-Scale, Cost-Effective, Reproducible Manufacturing of High-Quality Cells: A Technology Roadmap to 2025.
- Robinson, et al. Low temperature call pausing: an alternative short-term preservation method for use in cell therapies including stem cell applications. Biotechnol. Letters, 2014 Feb;36(2):201-2019.
