 As early as 1969 Shaw and Pace reported that: “Cells tolerate a low O2 exposure (5%) for at least 20 days while continuous exposure to high O2 atmosphere results in degeneration and death after 7–10 days” (1). These were one of the first experiments showing that cells in culture survive better in hypoxia although no explanation was conceived immediately. In the following 45 years more than 39,000 papers have been published bringing detailed data about how the cells respond to excess or shortness of Oxygen availability (PubMed). Culturing cells in low oxygen availability (hypoxia) has become the standard procedure in cell culture for research purposes or for pharmaceutical test processes, introducing in the regular cell culture laboratories different sorts of hypoxia bioreactors, hypoxia chambers and complex glove boxes. The progress in stem cell management and regenerative medicine applications even enhanced the need of culturing in hypoxic conditions or in Physiologically Relevant Oxygen Tension (2). The question is how much Oxygen is “physiologically Relevant”
As early as 1969 Shaw and Pace reported that: “Cells tolerate a low O2 exposure (5%) for at least 20 days while continuous exposure to high O2 atmosphere results in degeneration and death after 7–10 days” (1). These were one of the first experiments showing that cells in culture survive better in hypoxia although no explanation was conceived immediately. In the following 45 years more than 39,000 papers have been published bringing detailed data about how the cells respond to excess or shortness of Oxygen availability (PubMed). Culturing cells in low oxygen availability (hypoxia) has become the standard procedure in cell culture for research purposes or for pharmaceutical test processes, introducing in the regular cell culture laboratories different sorts of hypoxia bioreactors, hypoxia chambers and complex glove boxes. The progress in stem cell management and regenerative medicine applications even enhanced the need of culturing in hypoxic conditions or in Physiologically Relevant Oxygen Tension (2). The question is how much Oxygen is “physiologically Relevant”
Because of the recent new plethora of devices allowing easy spheroid cultures of combined cell types and matrix molecules, many scientist are infatuated with the 3D cultures, calling them Physiologically Relevant Cultures because, compared with monolayer cultures 3D clusters have broad intercellular contacts and cells are exposed to a gradient of oxygen tension with minimal O2 tension in the cells of the sphere core.
Actually the cells of spheroids, cultured in regular 21% oxygen, are exposed to a wide range of Oxygen tensions related to the diffusion gradient established within the surface cell layer and the center, depending on the spheroid size, the cell type and the individual oxygen consumption of each cell.
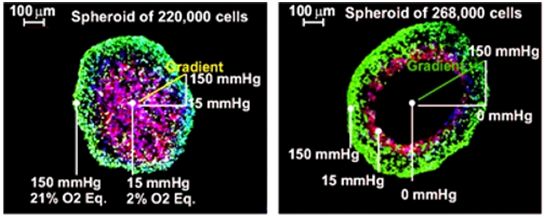
In spheres cell responses are heterogeneous, associated to oxygen availability progressive decay at specific topographic locations (3) (4). When 3D cultures are set up the amount of oxygen is not really controlled. Apparently the inner cells in the core of the spheroid will be exposed to physiologic Oxygen tension because the cell layers between the core and the shell will reduce the amount of available oxygen down to physiologic levels. But this is just a rough approach to the truth; the amount of available Oxygen for each cell included in a spheroid depends on the diameter of the sphere, which depends on the number and size of cells included in it, the cell type, cell cycle phase of each cell and the relative position of each cell in the sphere. Cells on the sphere surface (the shell) are exposed to the environment oxygenation, and cells in the core could be exposed to a broad range of Oxygen tensions (9) (10). As long as the media has 150 mmHg of Oxygen tension, depending on the spheroid diameter and the cell type, the inner cells may be over oxygenated like in traditional 2D cultures (flasks, dishes…) or completely anoxic (dead) (see figure 1). The average Oxygen tension for each cell in Spheres of 10,000 cells or less will be a minimum equivalent to 15% atmospheric Oxygen (100 mmHg). Only over 100,000 cells spheres will show gradients of hypoxia from the shell to the center of the core (see figure 1. Green cells Oxygen tension between 100 and 150 mmHg, Red cells Oxygen tension between 15 and 100 mmHg and dark cells below 15 mmHg). Therefore these 3D models need expert attention and may properly read intercellular surface interactions but will not represent in any case a physiologic average oxygen supply.
Hypoxic chambers and bioreactors have different qualities and limitations. In terms of biological limitations, simple 2D cell cultures, without scaffolds or matrixes, lack extensive intercellular surface contacts, however all cells are exposed to the same oxygen tension and expectedly all show homogeneous adaptive responses to oxygen tension. This could be apparently inconsequential but it is not: Biological knowledge is always statistically generated, assembled with universal assumptions based on statistical analysis of limited samples. Therefore homogeneous samples provide more significant statistical data and large samples deliver fewer errors than small samples. The interest in Oxygen availability on in vitro cell physiology has increased in the last 15 years worldwide, shown by the more than 3,000 researches published on cell changes induced by the exposure of the cells to non atmospheric amounts of Oxygen, either by excess (hyperoxia) or defect (hypoxia), compared to the less than 900 papers published in the entire XXth century.
Old cell culture standards overlooked the excess of Oxygen delivered to the cells, cultured in media exposed to regular 21% Oxygen atmosphere; many molecular pathways where ignored until the first experiments were made (5) culturing the cells in artificial atmosphere, with reduced Oxygen, which showed important regulatory pathways triggered by low availability of Oxygen (6) (7) (8). Nowadays we understand that many research results obtained with classic cell culture standards (21% Oxygen and 5% CO2) may be artifacts because the excess of oxygen could down regulate certain genes and up regulate others, even modify the molecular interaction results.
When the concept of Physiologically Relevant Cultures is claimed it is required to consider, in first place, the amount of oxygen that the specific cells may need, and that obliges to know beforehand which the physiologic oxygen conditions of the specific organ are. By knowing the physiologic oxygen environment of a cell type in vivo, in the organs, it is easier to set the percentage of Oxygen in the incubator atmosphere attempting to mimic in vitro the real tissue environment.
Hypoxia obtained with precise chambers can be a more accurate and global example of average cell physiology in any specific living tissue environment. The environmental Oxygen tension can be exactly set in these chambers and in a few hours the culture media of the cell culture devices dissolve the amount of oxygen that represents the physiologic environment, exposing all cultured cells to a homogeneous Oxygen tension.
There are different papers that divulge the level of Oxygen tension in cells of special organs, physiologic status of the organs and even detailed locations in the organs (see attached table). Based on these data the cell cultures, in 2D can be grown in media with controlled levels of dissolved Oxygen (DO) maintaining a true physiological oxygenation. A limitation of these chambers is that practically all work with a fixed preset level of Oxygen, are cumbersome and certainly tedious to handle. In an effort to improve the maneuverability of this technology autonomous controlled Physiologic Hypoxia Chambers have been created (PetakaG3 LOT). These chambers, been smaller than a microtiter plate, are designed to maintain the precision of the Oxygen supply, without electronics, wires, tubing or gas canisters, and facilitate the development of True Physiologic Oxygenation in cultures of many million cells, maintained in standard incubators. A very important advantage of these Physiologic Hypoxia Chambers is that never allow anoxia within 0 and 5,000 feet of altitude, therefore the cells remain alive as long as the media contains the essential nutrients, because as soon as the media reaches the minimum level of DO required by the specific cell type, automatically a negligible diffusion flow of atmospheric new Oxygen enters in the cell culture chamber maintaining the minimum dissolved Oxygen level constant within less than 2% fluctuation (± ≈0.5 mmHg). That allows accurate cancer experiments, preparing millions of tumor cells cultured in homogeneous physiologic hypoxia as the tumors are in vivo. Cells cultured in these chambers, in these accurate conditions can be transferred to high throughput testing procedures or injected in animals for in vivo evaluation of tumorigenesis and metastasis.
It is important to consider that extreme hypoxia, such as 1% Oxygen in the incubator is effective for triggering the HIF response independently of the cell type but does not correspond to real physiologic levels of Oxygen, only to a fake accidental asphyxia of the animal. In all published data the minimal level of Oxygen in a tumor or in a normal tissue (even hypoxic) was never below 9.8 mmHg or equivalent 1.4% in the incubator, for example medullar thymus and Hematopoietic Stem Cell Niches in the bone marrow (11). The average physiologic Oxygen environment in both normal and tumor tissues is about 31 mmHg (equivalent to 4.35% Oxygen in the incubator). The Oxygen level in the Blastocyst is about 24 mmHg (equivalent to 3.5% Oxygen in the incubator). Nevertheless, if 1% Oxygen in the incubator does not mimic the Physiologic environment is even worse the classic, obstinate and thoughtless incubation in 21% regular atmosphere which is in the best case 100% over the highest physiologic level (feeding mammary gland), and in the case of the breast cancer cells and central hepatocytes more than 1,000% over the physiologic levels (See black column of the table).
That enormous divergence of the physiologic respiratory conditions between classic cell culture and real physiologic tissues suggests that major cell regulation molecular pathways discovered in hyperoxic cell cultures should be honestly revised culturing the cells in Physiologic conditions.
Recently cell culture devices are improving (i.e. Chips) that facilitate the partial in vitro reproduction of tissue structures closer to the physiologic reality, except for the size limitations these are real physiologic in vitro reproduction of a few models (12) (13). The progress in these technologies is increasing and the approaches are more and more interesting.
REFERENCES
1. Shaw DH, Pace DM. Recovery of cells in vitro from the effects of hypoxia and hyperoxia. J Cell Physiol. 1969 Apr 1;73(2):119–24.
2. Stacpoole SRL, Webber DJ, Bilican B, Compston A, Chandran S, Franklin RJM. Neural precursor cells cultured at physiologically relevant oxygen tensions have a survival advantage following transplantation. Stem Cells Transl Med. 2013 Jun;2(6):464–72.
3. Grimes DR, Kelly C, Bloch K, Partridge M. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface. 2014 Mar 6;11(92):20131124.
4. Mueller-Klieser WF, Sutherland RM. Oxygen tensions in multicell spheroids of two cell lines. Br J Cancer. 1982;45:256–64.
5. Vexler AM, Litinskaya LL. Changes in intracellular pH induced by hyperthermia and hypoxia. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 1986 Mar;2(1):75–81.
6. Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp JoVE. 2011;(54).
7. Haque N, Rahman MT, Abu Kasim NH, Alabsi AM. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal. 2013;2013:632972.
8. Nakajima R, Takeda S. The efficient fabrication of corneal epithelial cell sheets by controlling oxygen concentration. Exp Eye Res. 2013 Nov;116:434–8.
9. Grimes DR, Kelly C, Bloch K, Partridge M. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface R Soc. 2014 Mar 6;11(92):20131124.
10. Mueller-Klieser WF, Sutherland RM. Oxygen tensions in multicell spheroids of two cell lines. Br J Cancer. 1982 Feb;45(2):256–64.
11. Parmar K, Mauch P, Vergilio J-A, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci. 2007 Mar 27;104(13):5431 –5436.
12. Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011 Dec;21(12):745–54.
13. Trietsch SJ, Israëls GD, Joore J, Hankemeier T, Vulto P. Microfluidic titer plate for stratified 3D cell culture. Lab Chip. 2013 Aug 13;13(18):3548–54
