The vast majority of cells in laboratory culture are grown in media equilibrated to the open atmosphere containing ~21% oxygen. Since most cells in the body are perfused at the equivalent of 3-7% oxygen, typical laboratory culture conditions inadvertently expose cells to 3-7 times higher oxygen levels than what is considered physiological.1 This hyper-aerobic culture condition can lead to major changes in cellular metabolism, oxidative stress levels, and gene expression compared to cells cultured under physioxic conditions (3-7% oxygen). 2 It can be no surprise then that most cell culture systems, no matter how sophisticated and elegantly engineered on a cellular level, fail to correctly predict biological activity in-vivo.
For cancer research, differences in oxygen levels during culture is especially significant, since most solid tumor cells are perfused at the equivalent of <2% oxygen.1 This hypoxic condition of tumor cells means that open air incubation delivers 20-40 times the levels in-vivo. Oxygen levels in tumor research is critical since it is well known that this natural hypoxic state confers tremendous resistance to tumor cells for both chemotherapy and radiation treatment. The end result is that most tumor lines and primary tumor cells grown in high oxygen will not predict a real physiological response to treatments in the laboratory, leading to many false positive results and clinical failures. This fact is important to keep in mind, not only for cell therapies, but also for emerging non-cell therapies like exosomes.
Exosomes are small extracellular vesicles of endosomal origin with a diameter up to 150nm. These particles are recognized as carriers of a broad range of cellular communication and signaling molecules like proteins, lipids and nucleic acids, including regulatory micro-RNAs. Recently, interest in exosomes has grown due to the discovery that they play a key role in intercellular communication, immunomodulation, and involvement in fibrosis, cancer, and neurodegenerative diseases, among other conditions.
Exosomes, particularly those generated from mesenchymal stem cells, have recently demonstrated promising therapeutic potential in myocardial infarction and other ischemic model systems via autophagic mechanisms,3 neoangiogenesis,4,5 promotion of macrophage M2 polarization,6 or an miR-210 mediated mechanism, 7 which may involve apoptosis signaling or a mitochondrial protection pathway. 8 Everywhere they are tested, exosomes seem to show strong promise as a direct therapeutic agent, diagnostic marker, or as a potential drug carrier.
Early research results have led to an explosion of exosome research, not only due to their ready-made therapeutic potential but also due to their relative ease of production and scalability.
However, as exosome production rapidly advances from translational research to the clinic, manufacturers will quickly find that exosomes manufactured under various conditions are not created equal. Recent findings have shown that the exosomes may differ in size, quantity, and molecular content when cells are exposed to various environmental stresses, including heat, oxidation, nutrient levels, genotoxic stress,9 and particularly changes in oxygen levels during culture.10
Hypoxic treatment of cardiac progenitor cells was shown to produce exosomes that enhance tube formation of endothelial cells, improve post-ischemic cardiac function, and reduced fibrosis in an ischemic-reperfusion cardiac injury model.11
In a wound model co-culture system, treatment with exosomes from hypoxia-stimulated pericytes (MSCs) resulted in faster wound healing, greater vascular density and greater endothelial cord formation compared to HIF-inhibited controls. 12
MSCs cultured under low oxygen produce exosomes with a higher angiogenic capacity, likely mediated through a Jagged-1 (Notch-mediated) signaling pathway. This and other results suggest that exosomes produced under low oxygen conditions may have more therapeutic potential for post-ischemic injuries, like MI and stroke.13
It is clear that tumor cell exosomes are critical in modulating the tumor microenvironment. Cancer cells in culture have been shown to produce more exosomes under hypoxic conditions than parental cells under normal, hyperoxic culture conditions. This is evidence that hypoxia, which is the tumors normal state, upregulates the baseline exosome production, and the subsequent paracrine signaling level. This is important because a recent review by Shao, et al. describes how exosomes induced by hypoxia and via HIF-mediated mechanisms participate in tumor invasion, metastasis and angiogenesis; all currently key targets for therapeutic research.14
In a recent study by Matsuura, et al, it was shown that hepatocyte carcinoma cells grown in low oxygen generated greater angiogenesis as measured by HUVEC tube formation following co-culture and that this enhanced angiogenesis potential was mediated by an upregulation of miR-155.15 This is an significant because miR-155 is known to be a critical signaling factor in pathologic fibrosis16 and cancers of the breast, lung, liver and lymphatic systems.17
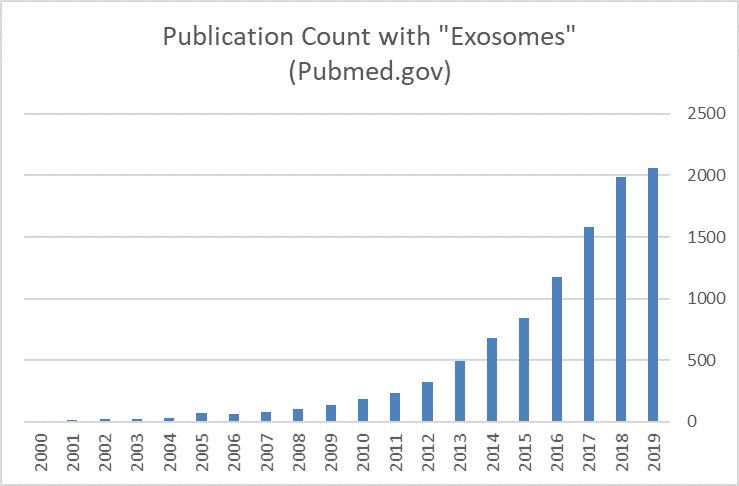
This summary is meant to highlight the importance of gas-control during exosome cell culture. To achieve low-oxygen levels during culture most facilities use tri-gas incubators or specialty environmental control equipment, however tri-gas incubators are expensive and need frequent canister replacement. Environmental control rooms and glove box set-ups are large and complex, they require maintenance, represent substantial capital expense. Therefore, researchers are looking for low-cost, efficient, and robust alternatives to achieve low-oxygen culture conditions. Currently, the only low-cost consumable on the market that provides precise, reliable and efficient low-oxygen culture is the Petaka G3™ cell culture cassette.

The Petaka G3™ is operated like a standard flask, and is therefore user friendly, but because it is functionally closed and features precise gas control it eliminates the need for expensive tri-gas incubators and glove box systems. So, if you ever want to explore the many benefits of “hypoxic” or physiologic cell culture but you don’t want to commit to large and expensive systems I recommend you try the Petaka G3™.
REFERENCES
- S R McKeown. Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br J Radiol. March 2014; 87(1035)
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012; 148: 399–408.
- Zou, et al. Bone marrow mesenchymal stem cell‑derived exosomes protect against myocardial infarction by promoting autophagy. Experimental and Therapeutic Medicine. 18: 2574-2582, 201.
- Xue, et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018 Apr 1;27(7):456-465.
- Han, et al. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J. Biochem Cell Biol. 2019 Apr; 109: 59-68.
- Deng, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019 Sep;114.
- Zhu, et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018 Dec;46(8):1659-1670.
- Bavelloni, et al. MiRNA-210: A Current Overview. Anticancer Research, Dec, 2017, 37;12: 6511-6521.
- Abramowicz, et al. Different Types of Cellular Stress Affect the Proteome Composition of Small Extracellular Vesicles: A Mini Review. Proteomes 2019, 7; 23: 1-10.
- Cosme, et al. Hypoxia-Induced Changes in the Fibroblast Secretome, Exosome, and Whole-Cell Proteome Using Cultured, Cardiac-Derived Cells Isolated from Neonatal Mice. J Proteome Res. 2017 Aug 4;16(8):2836-2847.
- Gray, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015 Jan 16;116(2):255-63.
- Mayo and Bearden. Driving the Hypoxia Inducible Pathway in Human Pericytes Promotes Vascular Density in an Exosome Dependent Manner. Microcirculation. 2015 November; 22(8): 711–723
- Gonzalez-King et al. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells. 2017 Jul;35(7):1747-1759.
- Shao, et al. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer, 2018 Aug 11;17(1):120.
- Matsuura, et al. Exosomal miR-155 Derived from Hepatocellular Carcinoma Cells Under Hypoxia Promotes Angiogenesis in Endothelial Cells. Dig Dis Sci. 2019 Mar;64(3):792-802.
- Eissa and Artlett. The MicroRNA miR-155 Is Essential in Fibrosis. Non-coding RNA 2019, 5, 23.
- Higgs and Slack. The multiple roles of microRNA-155 in oncogenesis. J. Clin Bioinformatics, September, 2013, 3(1):17.
