Phisioxia is a relatively new word that defines the amount of oxygen available for cellular respiration in normal animal tissues, especially in the tissues of mammals, because man is one of them and is the main subject of biological research.
For more than a century mammalian cells have been cultured in vitro in an aqueous medium with a large surface area exposed to the ambient atmosphere, which at sea level exerts a pressure of about 760 mmHg. Therefore, in many laboratories the atmospheric pressure is of a bar or 100 kPa, with an oxygen proportion of approximately 21%. Cultures of mammalian cells are usually done at a temperature of 37 ° C. These physical conditions lead to an oxygen saturation of the water, in the culture medium, of about 6.4 mg / L, which is equivalent to a partial pressure of oxygen (PO2) of about 150 mmHg. This pressure is much higher than that existing in the most of the tissues of the corresponding animals, which oscillates around 40 mmHg. That is to state that in cell cultures, which try to reproduce the natural environment (adjusted pH, osmolarity and nutrients) neglect the concentration of O2, the gaseous element fundamental for life on Earth, exposing the cells to more than 300% of oxidative overdose
The effect of that an excess of oxygen can cause, has been studied with different types of cells exposed to PO2 of up to 720 mmHg, an 1800% of what usually exists in the tissues of origin. In some of these investigations (1), it has been proven that certain cells can maintain an exponential growth even with a PO2 of 380 mmHg, almost 10 times the normal in tissues (Fig 1-A). However, the doubling time is lengthened (fig. 1A). In the aforementioned research they perform the same tests on two cell types, one of lymphoid origin (TK6 cells) cells that grow in suspension, and another of cells, of epithelial origin (VERO cells), which grow anchored to the surface of the cell culture device. The cells that grow suspended seem more resistant to the excess of oxygen, in fact; they tolerate up to oxygen concentrations equivalent of 720 mmHg, although these only survived 3 days, (Fig. 1-A. TK6 cells, purple diamonds). The epithelial cells, on the other hand, grow very little with excess oxygen, but they remain viable more days, 7 days with oxygen tensions of 570 mmHg (Fig 1A, VERO cells, black boxes).
Figure 1. Effects of both extremely high and low Oxygen availability on cell growth (Data extracted from references 1 and 4)
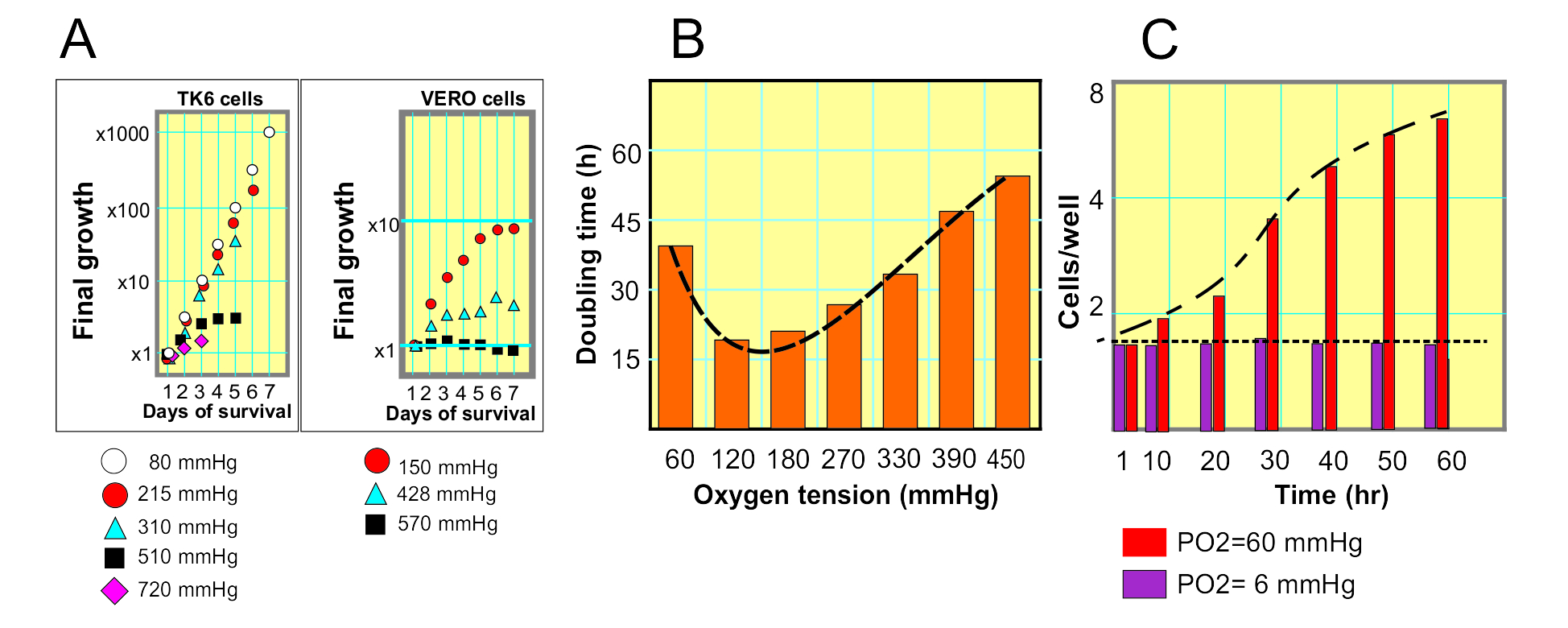 That research (1) also showed that both the excess and the deficiency of oxygen in the medium slow down the growth of cell populations (Fig. 1B). There is therefore an effect of hormesis in the cellular response to the amount of environmental oxygen, which can be added to the multiple biological and evolutionary effects in which the phenomenon of hormesis is manifested (2).
That research (1) also showed that both the excess and the deficiency of oxygen in the medium slow down the growth of cell populations (Fig. 1B). There is therefore an effect of hormesis in the cellular response to the amount of environmental oxygen, which can be added to the multiple biological and evolutionary effects in which the phenomenon of hormesis is manifested (2).
At the opposite experimental concept are the studies of Shrieve et al. (3) Testing the effect of extremely low oxygen on cell growth, which showed that the absence of oxygen stops the proliferation of cells (Fig. 1C).
These studies, with many others, highlighted the importance of the available oxygen in the living environment of the cells, as a component of crucial physiological importance for those who use cultured cells in research and industry. As a consequence, many cell biology research centers have been equipped with instruments with technology aimed at providing a cell culture environment with controlled oxygen, to achieve the desired PO2 in the culture medium. With this objective, a new saga of culture protocols in environmental hypoxia has been produced.
Most instruments developed to grow cells with limited oxygen, use methods to generate “environmental hypoxia” (such as the hypoxia chambers), not the “culture media hypoxia”. These consist of hermetic chambers where the culture flasks and plates are placed, and then gases are injected into the chamber from external tanks to alter the oxygen concentration in the chamber. The gases can be pure oxygen, nitrogen or a mixture of both at a pre-set concentration, and an O2 environmental sensor control when the gas inlet valves open or close. When the desired oxygen concentration in the chamber environment has been balanced, the final PO2 in the medium is decided by the diffusion of the gases into the medium, which progresses autonomously according to the environmental concentration of the gas, the temperature, the composition of the medium, the contact area between the environment and the cell culture medium, the quantity and class of the cultured cells. After a variable time, the medium will contain a concentration of gases proportional to the combination f all these variables.
The so-called tri-gas incubators act the same: Their chamber is hermetically sealed and they have oxygen, nitrogen and CO2 canisters, connected to inlet valves, controlled by Oxygen and CO2 sensors.
The use of these devices is easy, although relatively tedious. The most difficult of these systems is to maintain oxygen levels stable for long periods of time (days), and how to handle the cultures while maintaining the composition of gases. These handling maneuvers require that the hypoxia chambers be like glove boxes, with airlocks to keep the indoor disconnected from the outside environment.
However, conceptually, what is actually needed is to control the minimum level of oxygen required for maintaining the normal physiological activities of the cells, like in a specific differentiated tissues (specific physiologic minimal oxygen demand or SPMOD), a very important factor for normal and cancer biology research.
The aforementioned instruments refer back to what is programmed by an external operator that can decide on a scale of gas concentrations in the chamber or incubator environment, outside the culture medium, but does not directly control the concentration of gases dissolved in the actual biological ambient, the medium where the cells live. Moreover, if the SPMOD is unknown, setting the precise oxygen concentration of the incubator, or the hypoxia chamber, is just a loose random attempt.
Figure 2. Effect of Oxygen partial pressure on cell growth
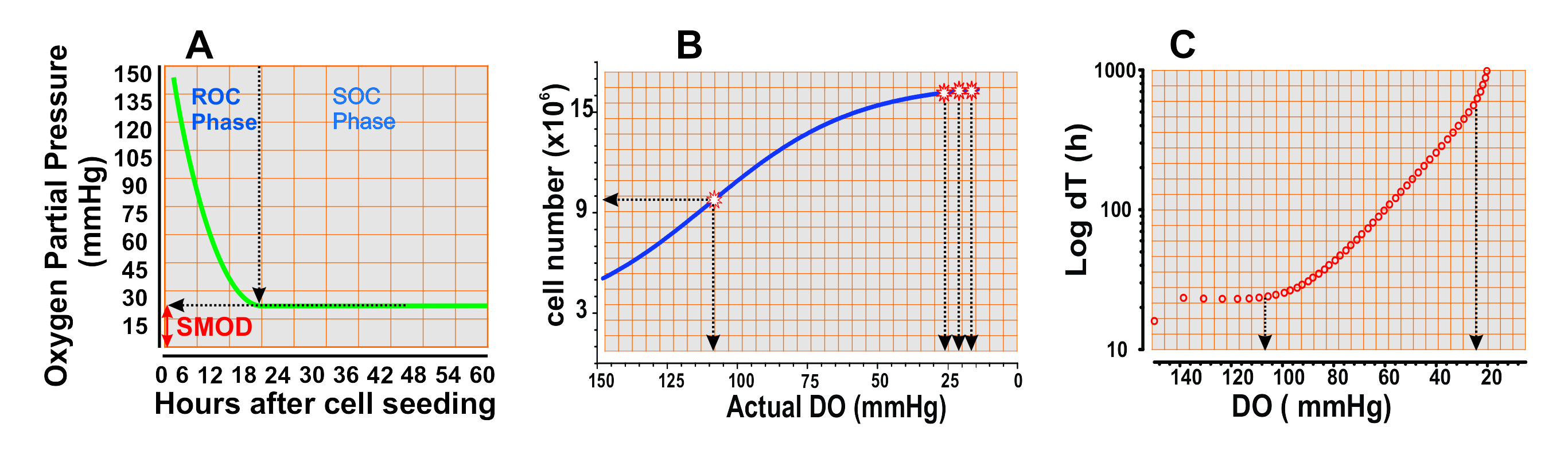 Nevertheless, cell culture in “Limited Oxygen” (LOCC) is safe, reliable and repeatable using “Ducted Respiratory Chamber Bioreactors” (4), such as Petaka G3 bioreactor, a virtually hermetic device that allows the passive, but restricted, diffusion of oxygen from the environment into the medium where the cells live, and meanwhile controls the CO2 run off from the the medium to the environmental atmosphere, ensuring the pH control with a bicarbonate buffer without external N or CO2 supply.
Nevertheless, cell culture in “Limited Oxygen” (LOCC) is safe, reliable and repeatable using “Ducted Respiratory Chamber Bioreactors” (4), such as Petaka G3 bioreactor, a virtually hermetic device that allows the passive, but restricted, diffusion of oxygen from the environment into the medium where the cells live, and meanwhile controls the CO2 run off from the the medium to the environmental atmosphere, ensuring the pH control with a bicarbonate buffer without external N or CO2 supply.
In this system, the diffusion velocity of O2 from the environment to the medium is below the average consumption of O2 of the internal population of cells, that condition determines a progressive depletion of O2 in the medium with an increase in the difference in partial pressures of O2 between the medium and the external atmosphere (Figure 2A). When the partial pressure of O2 of the medium “M” (PO2-M) exceeds that of the external environment “A” of the Petaka G3 (PO2-A), the increase of that difference accelerates the entry of O2 into the device and increase its diffusion in their medium [MORE INFO]. When the input speed is equal to the consumption of the cells, the -input ends and there is no output. If the cells continue to consume oxygen until the PO2-M is lower than PO2-A in the environment, then more O2 enters the medium again. The level of O2 solution in the environment is regulated by the cell population itself and the solid and simple diffusion laws proposed by Adolf Fick in 1855.
Petaka is completely autonomous, portable and can be manipulated in any environment without altering the O2 concentration of the medium, whatever the concentration of the environmental O2, as long as the cells growing in it stay alive and active..
The CO2 dissolved in the medium is regulated in the same way. The cell population generates CO2 with its metabolism; If the partial pressure of CO2 in the medium (PCO2-M) is greater than the atmospheric one, it will escape to the atmosphere, if it will not be contained in the medium up to a level compatible with the common bicarbonate buffer, which makes it unnecessary to increase the concentration of CO2 in the incubator atmosphere.
So, for culturing the cells in Euphysioxia, the procedure with Petaka G3 DO-sensor is straightforward:
- Fill the Petaka with the appropriate medium for the cell type.
- Let the cells grow at 37O Celsius
- Measure the dissolved Oxygen automatically every 15 seconds
- After 40 hours of culture, analyze the curve of the DO progression, recording the data in an Excell file with the NeoFox software.
- Analyze the data. It will be evident a phase of rapid DO loss (Fig 2A), or Reckless consumption of O2 (ROC phase), when the dissolved oxygen in the medium is in between 30 mmHg and 140 mmHg, which last in this specific experiment about 10 hours since the seeding of 5 million HeLa cells in 25 mL of oxygen saturated medium, about 150 mmHg (Fig 2, A). These early phase is followed by a phase of flat growth progression (Fig 2 B and C), a phase of sustainable consumption of O2 (SOC phase). In this phase the global cell growth is progressively decelerated (fig 2 B and C), showing a certainly dramatic increasing of the doubling time, since 110 mmHg (Fig 2 C). The second derivative of the growth function discloses a critical inflection point about 110 mmHg bellow which cells decelerate the growing rate (the growth curve changes from a concave shape to a convex shape, Fig 2 B star). A second critical point is visible after an average value of DO of 20 to 30 mmHg; after this point the second derivative of the growth curve shows a wavy progression with multiple inflection points and small amplitude waves (Fig 2C stars), consequence of the feedback interactions between the O2 consumption of the cells and the limited O2 diffusion, regulated by the Petaka G3 bioreactor system.
Along this, in 1-2 hours of this responsive phase will show the amplitude of SMOD of this specific cell type in the specific medium and conditions, such as temperature, pH and barometric pressure (Fig 2A red arrows).
REFERENCES:
[1]Oller A.R., Buser, C.W., Tyo, M.O., Thilly, W.G.. 1989. Growth of mammalian cells at high oxygen concentrations. Journal of cell science, jcs.biologists.org [2] Calabrese, E.J., Mattson, M.P., 2017. How does hormesis impact biology, toxicology, and medicine? npj Aging and Mechanisms of Disease. volume 3, Article number: 13 [3] Shrieve, D.C., Deen, D.F., Harris, J.W. 1983. Effects of Extreme Hypoxia on the Growth and Viability of EMT6/SF Mouse Tumor Cells in Vitro. Cancer Research 43, 3521-3527. [4] J. Gallagher, J.,Barbera-Guillem, E., 2012. Ducted Respiratory Chamber Bioreactors. GEN. 32, 19.2012.