Like most discoveries, the stem cell concept was not the result of a single research. The concept was built in years, as an integrated understanding of multiple results, apparently not linked. Around 1880, W. Roux presented experiments in which early embryos were spliced in half, showing that parts of an embryo, separately failed in developing a complete fetus. In 1891, In Freiburg (Germany), Eduard Driesch succeeded in separating the cells of the first cleavage of a sea urchin egg and growing from them identical twins (1). This result allowed him to sustain the theory of “cell pluripotency”, meaning that each cell retained the ability to develop an entire organism. Also, in Germany, in 1909, Artur Pappenheim collected enough experimental data to sustain the theory of the existence of a “Blutstammzellen”, the origin of all types of blood cells. Much later, in 1936, Florence R. Sabin, from the Rockefeller Institute (USA), studied the development of the rabbit bone marrow proving the existence of a precursor cell population, with undefined morphology, similar to lymphocytes, able to develop all blood cell lines (2). In 1958, in France, Marcel Bessis demonstrated that the blood stem cells need interaction with stromal cells to differentiate in a red blood cell colony (3). In 1961, Till & McCulloch showed that the hematopoietic stem cells are sensitive to radiation (4). These results consolidated the technical feasibility of stem cell elimination and substitution, the foundation of the bone marrow transplant, and probably the first application of regenerative medicine initiated by E.D. Thomas.
But the self-renewal nature of the hematopoietic stem cell, although it was suspected, was not experimentally proven until 1968 (5). Surprisingly, from 1900 until 1970 only 129 papers are recorded in PubMed archives including the linked two words “stem cell”, mainly oriented to hematopoiesis. This period merged the concept of the existence of “Stem Cells” in adult tissues, like bone marrow, capable of developing many different branches or lineages of cells (differentiated cells). Those stem cells were capable of auto-renewal following a stochastic process (6). Adding all different concepts together the attribute of “stem” was reserved for cells capable of developing “branches” by differentiation like the paradigmatic bone marrow stem cell.
Because a fetus has a broad variety of cells, all derived from the egg, this cell is by definition the first Stem Cell or even more, the “all potential” cell (Toti-Potent Cell). The first embryonic cells, because they have multiple or plural potentials, they are considered “Pluri-Potent Cell”
Bone marrow and hematopoiesis were excellent targets for tissue regeneration research, but another tissue, the liver, was also appealing and eye-catching for multiple reasons. Nobody knows how the ancient Greeks, figured out that the liver could regenerate after heavy tissue losses. But certain knowledge had because they create and spread the myth of Prometheus; who was punished by Zeus The punishment was to be chained to a rock, and every day an eagle would eat part of his liver, which will regenerate overnight.
Since the beginning of the 20Th Century, liver regeneration was a well-known fact mainly studied in rodents. The liver is endoderm derived tissue composed of several cell types with two major specific components: Hepatocytes and bile duct cells. Studies on the liver tumor development suggested that both cell types derive from a single stem cell, and this was identified as the “Oval Cell”, visible fundamentally in the liver of rodents, apparently located in the structure of the Hering channel (7). As soon as 1947 it was observed that liver regeneration was enhanced in hypoxic conditions (8) providing one of the first clues that the amount of oxygen has a cell regulation role in tissue regeneration (9).
However, these cells were considered more in the class of the “Reserve Cells” which the classic histologists use to find in the skin epithelium (10), in the intestinal cryptal epithelium (11), in the uterine mucosa (12), striated muscle (satellite cells) (13) and others, that many people today also called stem cells.
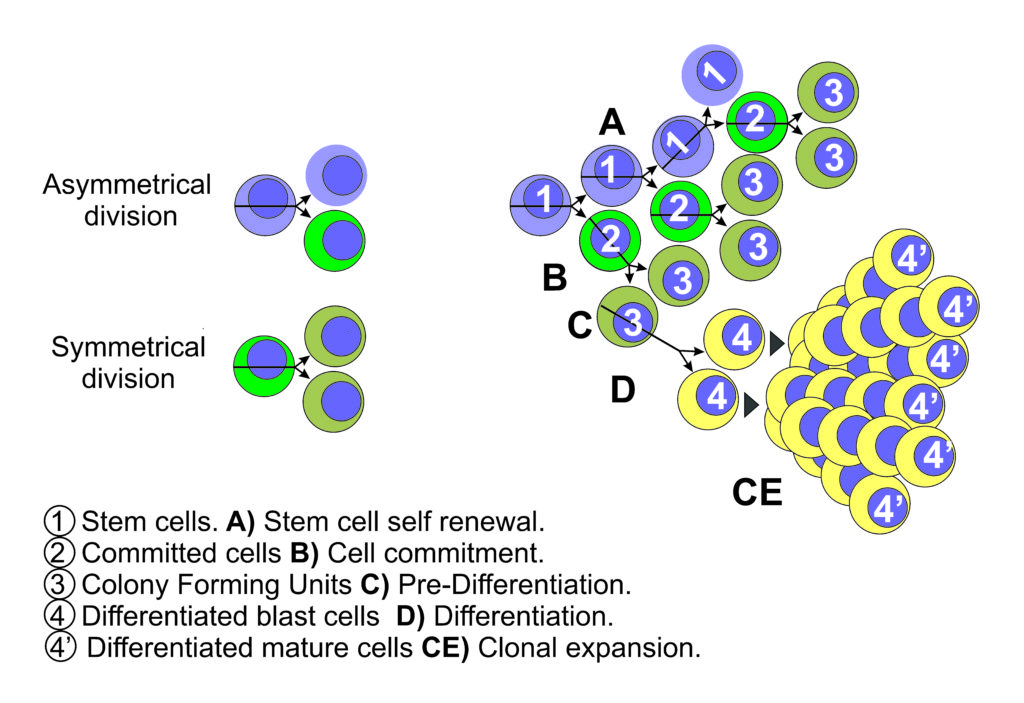
Together with the concept of stem cells, it arises the concepts of asymmetric and symmetric cell division. Cells divide asymmetrically when producing two cells with different destiny, and symmetrically when they produce two cells with identical destiny. It was deemed that asymmetric cell division is a crucial characteristic of stem cells enabling them both, to perpetuate themselves (self-renew) and generate differentiated progeny (14).
Reserve cells are capable of cell renewing by asymmetric division (15), and able to develop or differentiate in a limited variety of local cells, but they are not pluripotent, they are already “committed” to a certain cell type differentiation, therefore if they are considered or called stem cells, these are a limited type of stem cell, that may have the ability of limited asymmetric division period, followed by asymmetrical division life that produces a progeny of differentiated cells (Fig 1).
The unanswered questions about the pluripotent cells, and these limited-potent cells, as if they contained all genes involved in the development of a complete being, or if the differentiation process involved gene losses or genes definitely blocked. In 1958, J.B. Gurdon provides an experimental solid answer by generating frogs from a un-nucleated ovule in which he transplanted the nucleus of well-differentiated intestinal epithelial cells (16), therefore the nucleus of a differentiated cell contains all genes needed for the development of a complete being (17). In addition, this experiment demonstrated that the egg cytoplasm contains factors than can “reprogram” the genetic expression of the differentiated nucleus up to a pluripotent cell nucleus. In 1988, Henry Harris summarized his research on heterokaryons demonstrating that even the tumor cells can be reprogrammed when in the cytoplasm exist certain “suppressor genes” (18). Concise research opened another line of thinking: The differentiation involves the “sequential expression” of different genes (19). These concepts open the theoretical possibility of reprogramming by “altering the sequence” of the gene expression.
The beginning of an effective cell therapy started with the bone marrow transplant primarily developed by ED Thomas (20) in 1959. This valuable medical application invited to extend research on the concept and use of the stem cells (note that from 1970 to 1998 there are recorded in the PubMed archives about 3,000 publications involving stem cell matters).
Human pluripotent cell lines derived from blastocyst were first described and published in 1998 by J. Thomson et al. The authors describe them as cells with normal karyotypes, expressing high levels of telomerase activity, cell surface markers that characterize embryonic stem cells and markers which are lost in other cells of early lineages (21). Moreover, these cells can proliferate in vitro, maintaining the developmental potential to form derivatives of all three embryonic germ lines (22), meaning that they can divide symmetrically for quite a broad expansion.
Molecular biology studies identified active molecules with different regulatory functions that identified pluripotency and those become the basic instrument to identify stem cells, pluripotency traits and differentiation. Between all of them, a few are really consistent (Oct4, Klf4, Sox2, NANOG, and c-Myc).
In 2006 Takahashi K, Yamanaka demonstrates that adult fibroblast nuclei can be reprogrammed into embryonic stem cell phenotype by introducing in them a set of specific factors (Oct3/4, Sox2, c-Myc, and Klf4). These are the induced pluripotent stem cells (IPSC) (23). However, the iPS cells do differ from the embryonic stem cells ESC in the gene expression and DNA methylation patterns, but the additional expression of NANOG transcription factor results in germline-competent iPS cells with ES-cell-like gene expression and DNA methylation patterns (24). Thus, iPS cells competent for the generation of germline chimeras can be obtained from differentiated cells, such as fibroblasts, with today established tools like OKSM which consists of four adenoviruses expressing one of four mouse transcription factors (Oct4, Klf4, Sox2 or cMyc) under the control of a promoter. However, the retroviral introduction of c-Myc is dangerous for clinical application (25) cutting short the possible clinical application. It is also possible to reprogram amniotic fluid stem cells, even more easily than adult fibroblast using ectopic expression of OKSM. Nevertheless, the iPS cells are a great promise and represent extraordinary progress in the research and knowledge of cell differentiation and tissue regeneration.
Cancer is a major research and practice field where stem cells are a mystery. Many people are thinking that like any other tissue the neoplastic tissues may have reserve cells capable of self-renewal. It has been for a long time admitted that malignant tumors shed cells that are transported to other sites to reproduce the same neoplastic tissue in different organs (metastases). From this point of view, there is no doubt that these tumors have at least “reserve cells”, better called “metastatic cells” that have a specific phenotype and can reproduce the tumor from one or a few cells. For more than 40 years, it has been well known that cancer and metastatic tumor cells can show expression of fetal/embryonic genes (26, 27) as a sign of retro differentiation (28). Recently the “stem cells” are fashionable and there is in the journals a plethora of papers (29) claiming the finding of cells that they call “stem cells” because, as shown 30 years ago, they are cells that express one or more retro differentiation genes. Many prudent people call these cells, tumor colony-forming cells, “cancer stem-like cells” (CSCs) or “tumor-initiating cells”, which is closer to how they have been considered for years. However, to be “stem” is not only a matter of showing active embryonic genes. To be STEM means that the cell is capable of both self-renewal and differentiation. A metastatic cell is capable of self-renewal, of course, but it is not clear if it is capable of developing stable branches or lineages of cells with specific functions and traits. Many scientists claim that tumors are formed by a variety of different cells with different traits and those are enough to confer the honor of STEM to the tumor-initiating cells (30). However, it is not yet clear if each group of cells of this heterogeneous tumor cell population can be considered a branch or a linage with consistent differentiation (31). The evidences are in better agreement with very unstable clonal variations due to division errors, DNA damage, replication errors, immune editing and responses to different stressing environments (32). A cell lineage or a branch of stem cells requires a stochastic process of setting on and off a collection of genes, under a program existing in the egg cell. For it is known of the oncogenesis, it is not clear that cancer cells are following a well-defined program of differentiation, on the contrary, it looks like they have lost the control of the genetic program inherited from the normal stem cell or the tissue reserve cells.
REFERENCES
1. Driesch, Hans. Entwicklungsmechanische Studien: I. Der Werthe der beiden ersten Furchungszellen in der Echinogdermenentwicklung. Experimentelle Erzeugung von Theil- und Doppelbildungen. II. Über die Beziehungen des Lichtez zur ersten Etappe der thierischen Form-bildung. Z. Für Wiss. Zool. 1891;53:160–84.
2. Sabin FR, Miller FR, Smithburn KC, Thomas RM, Hummel LE. Changes in the bone marrow and blood cells of developingrabbits. J. Exp. Med. 1936 Jun 30;64(1):97–120.
3. BESSIS M. [Erythroblastic island, functional unity of bone marrow]. Rev. Hématologie. 1958 Mar;13(1):8–11.
4. Till JE, McCulloch EA. A Direct Measurement of the Radiation Sensitivity of Normal Mouse Bone Marrow Cells [Internet]. Radiat. Res. Off. J. Radiat. Res. Soc. 2010 [cited 2013 Jul 4]. Available from: http://www.rrjournal.org/doi/abs/10.2307/3570892
5. Vogel H, Niewisch H, Matioli G. The self renewal probability of hemopoietic stem cells. J. Cell. Physiol. 1968 Dec;72(3):221–8.
6. Matioli G, Niewisch H, Vogel H. Stochastic stem cell renewal. Rev. Eur. Détudes Clin. Biol. Eur. J. Clin. Biol. Res. 1970 Jan;15(1):20–2.
7. Vestentoft PS. Development and molecular composition of the hepatic progenitor cell niche. Dan. Med. J. 2013 May;60(5):B4640.
8. Drabkin DL, Lecrone W. Liver regeneration and Cytochrome c metabolism. Influence of anoxia and of injection of Cytochrome c. J. Biol. Chem. 1947 Nov 1;171(1):409–17.
9. Zeuthen E. Cell division and oxygen consumption in the eggs of Urechis caupo; experiments with modified Cartesian divers. Anat. Rec. 1948 Aug;101(4):732.
10. Cangkrama M, Ting SB, Darido C. Stem Cells behind the Barrier. Int. J. Mol. Sci. 2013;14(7):13670–86.
11. Wright NA. Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferative units and cancer. Int. J. Exp. Pathol. 2000 Apr;81(2):117–43.
12. Morelli SS, Yi P, Goldsmith LT. Endometrial Stem Cells and Reproduction. Obstet. Gynecol. Int. [Internet]. 2012 Jan 12 [cited 2013 Jul 7];2012. Available from: http://www.hindawi.com/journals/ogi/2012/851367/abs/
13. Montarras D, L’honoré A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. Febs J. 2013 Jun 5;
14. Caussinus E, Hirth F. Asymmetric Stem Cell Division in Development and Cancer. In: Macieira-Coelho A, editor. Asymmetric Cell Div. [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007, p 205-225.
15. Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006 Jun 29;441(7097):1068–74.
16. Gurdon JB, Eldsdale TR, Fishberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958 Jul 5;182(4627):64–5.
17. Gurdon JB. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J. Embryol. Exp. Morphol. 1960 Dec;8:505–26.
18. Harris H. The Analysis of Malignancy by Cell Fusion: The Position in 1988. Cancer Res. 1988 Jun 15;48(12):3302–6.
19. Flickinger RA. Sequential gene action, protein synthesis, and cellular differentiation. Int. Rev. Cytol. 1962;13:75–98.
20. Thomas ED, Lochte HL Jr, Canon JH, Sahler OD, Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. J. Clin. Invest. 1959 Oct;38:1709–16.
21. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM.. Embryonic stem cell lines derived from human blastocysts.. Science 1998 Dec 4;282(5395):1827.
22. Hamazaki T, Oka M, Yamanaka S, Terada N. Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation. J. Cell Sci. 2004 Nov 1;117(Pt 23):5681–6.
23. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76.
24. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007 Jul 19;448(7151):313–7.
25. Spinelli V, Guillot PV, De Coppi P. Induced pluripotent stem (iPS) cells from human fetal stem cells (hFSCs). Organogenesis. 2013 Apr 1;9(2).
26. Busch H. A General Concept for Molecular Biology of Cancer. Cancer Res. 1976 Nov 1;36(11 Part 2):4291–4.
27. Adinolfi A, Adinolfi M, Lessof. Alpha-feto-protein during development and in disease. J. Med. Genet. 1975 Jun;12(2):138–51.
28. Klein G. Advances in cancer research. Academic Press; 1979.
29. Shimono Y, Ugalde MZ, Cho RW, Lobo N, Dalerba P, Qian D, et al. Down-regulation of miRNA-200c Links Breast Cancer Stem Cells with Normal Stem Cells. Cell. 2009 Aug 7;138(3):592–603.
30. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, et al. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006 Oct 1;66(19):9339–44.
31. Denysenko T, Gennero L, Juenemann C, Morra I, Masperi P, Ceroni V, et al. Heterogeneous phenotype of human glioblastoma. In vitro study. Cell Biochem. Funct. 2013 Jul 8;
32. Polyak K. Heterogeneity in breast cancer. J. Clin. Invest. 2011 Oct 3;121(10):3786–8.
33. Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003 Jan; 120 (1): 117–30.
