
Established in 2002, Celartia Inc., is a biopropcessing technology company based in Columbus, Ohio. Celartia integrates the world’s only autonomous environmental control system into a single-use bioprocess disposable, the Petaka G3™. Successfully commercialized for over a decade, the performance of the Petaka G3™ is proven, with many academic laboratories, commercial biotechs, and CMOs relying on it to meet their clinical manufacturing and research needs. Celartia is currently developing the world’s first, fully automated cell manufacturing system around the Petaka G3™ cassette, dubbed the CCAL™.
Petaka G3 Published References
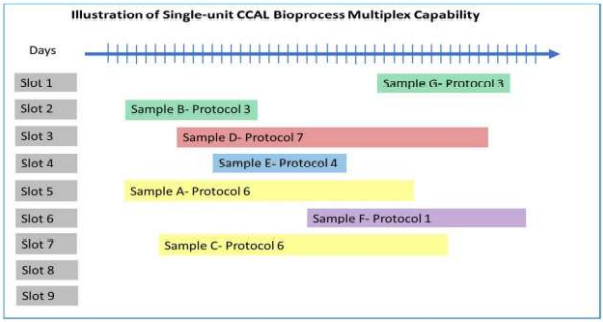
The CCAL™ is the world’s first and only high-density, compact, end-to-end automated, GMP-ready bioprocessing system. One CCAL™ will simultaneously process up to 400 unique autologous patient samples, running multiple unique protocols, in a workstation about the size of a biological safety cabinet. A single CCAL™ is sufficient to process up to 10,400 engineered GMP cell doses per year, requiring no more than three full time human operators. The output of a single CCAL™ will be sufficient to support a large and active clinical center of excellence, while reducing total production cost by 80-90% compared to current manual and semi-automated GMP production.
CCAL™ Project Highlights
- The Petaka G3™ was designed to be utilized in a fully-automated cell processing system
- The CCAL™ will process up to 400 unique patient samples, executing a variety of protocols in parallel
- Critical CCAL™ technology has been manufactured
- SBIR Phase 1 feasibility testing of proprietary CCAL™ technology has been completed
- The core CCAL™ automation technology has been de-risked from functional perspective
- Work continues with pending SBIR Phase 2 contract and other support to deliver prototype
- The CCAL™ has already been recognized as a 2023 Hello Tomorrow, Deep Tech Pioneer Award winner
- Current Technology Readiness Level (TRL) of 6/7, with a TRL of 7/8 at end of Phase 2 (Q2/Q3-2024)
- Celartia, Inc will be prepared to launch CCAL™ into the multi-billion cell and gene therapy manufacturing market with a product launch in 2025
Celartia invites potential finance, technology, and commercial partners to contact us: info@celartia.com
